US control trial: diabetic gastroparesis1
Objective
This trial examined the safety and efficacy of Enterra Therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis of diabetic etiology.
Design
Prospective, multi-centres, double-blinded, 1:1 randomised controled trial with cross-over study design across 8 centers in the United States.
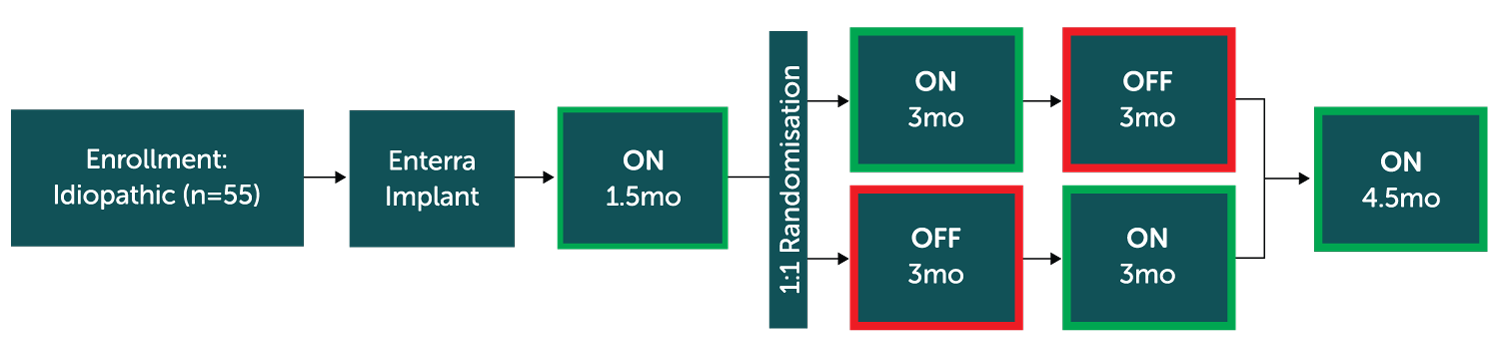
Endpoints
Weekly Vomiting Frequency (WVF); post crossover ON phase relative to OFF phase.
Results
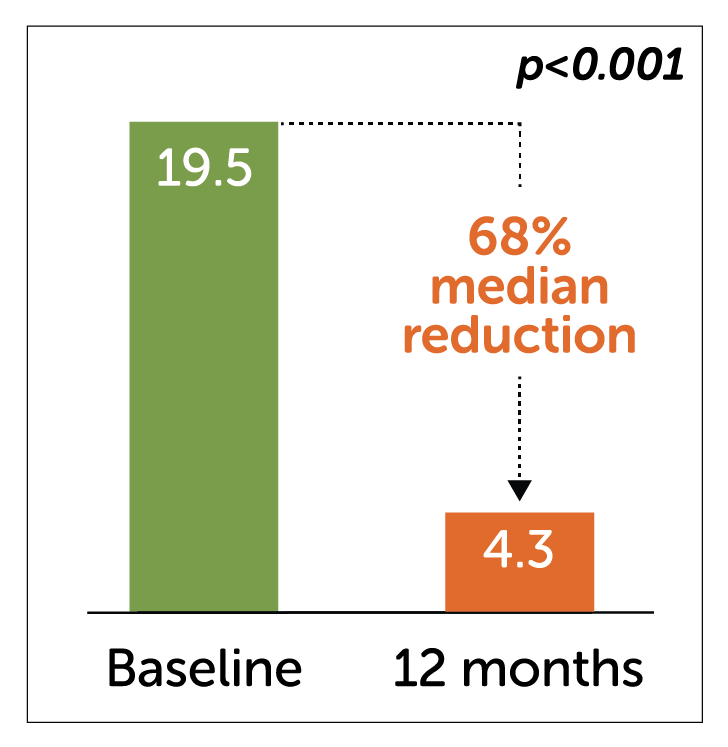
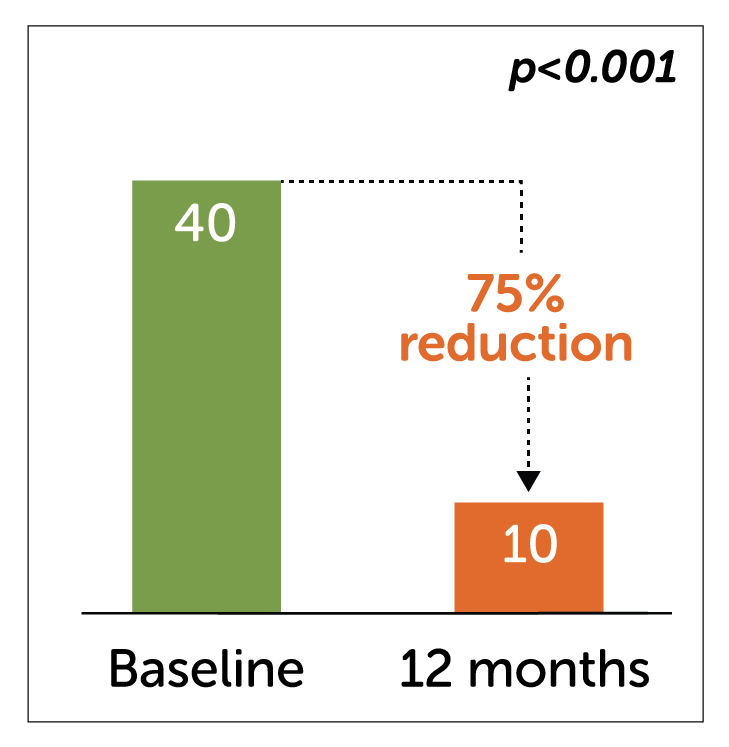
- In a study of 55 diabetic gastroparetic patients, Enterra Therapy significantly reduced median WVF at 12 month follow-up.
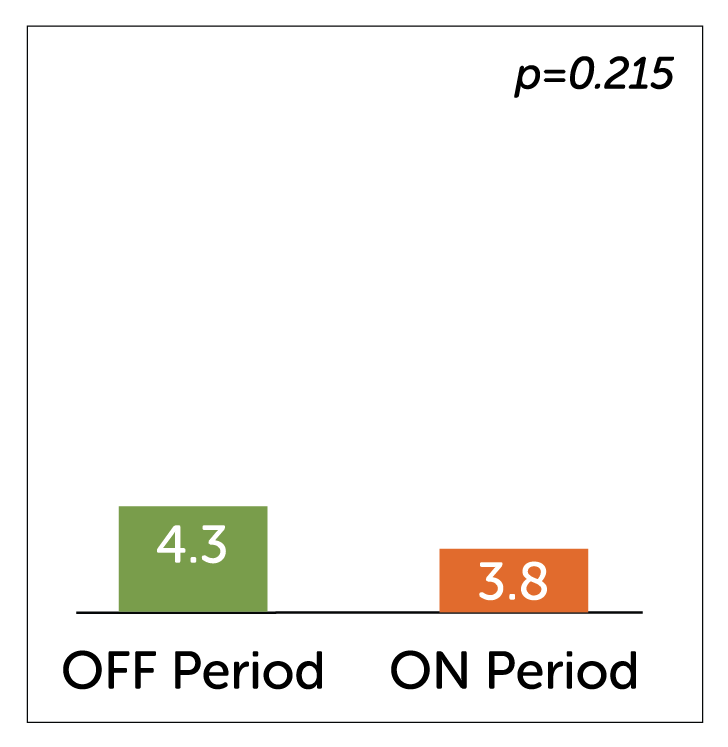
- Crossover periods showed a non-significant reduction in vomiting in the ON vs. OFF period which may have been due to the lack of washout effect of 6 weeks of stimulation that took place pre-randomisation.
- Enterra Therapy significantly reduced other key endpoints of total symptom scores and number of days hospitalised at 12 months.
Median Weekly Vomiting Frequency (WVF)
Total symptom score – severity
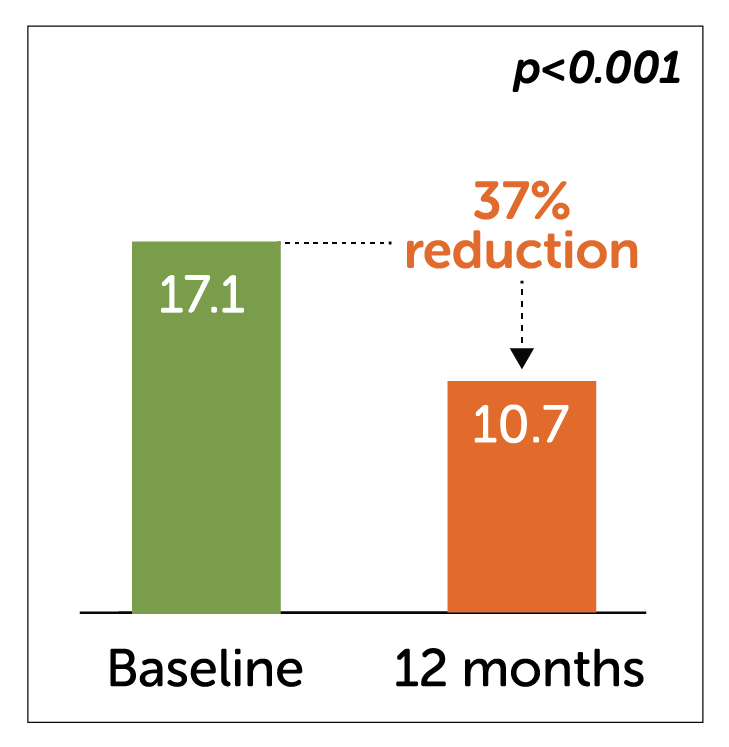
Hospitalization days
- McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8(11):947-e116. doi:10.1016/j.cgh.2010.05.020.
